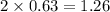At a certain temperature, the equilibrium constant, K c , for this reaction is 53.3. H 2 ( g ) + I 2 ( g ) − ⇀ ↽ − 2 HI ( g ) K c = 53.3 At this temperature, 0.800 mol H 2 and 0.800 mol I 2 were placed in a 1.00 L container to react. What concentration of HI is present at equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2


Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1
You know the right answer?
At a certain temperature, the equilibrium constant, K c , for this reaction is 53.3. H 2 ( g ) + I 2...
Questions









Mathematics, 22.12.2021 14:00


Physics, 22.12.2021 14:00

SAT, 22.12.2021 14:00

SAT, 22.12.2021 14:00

Mathematics, 22.12.2021 14:00



Mathematics, 22.12.2021 14:00



Chemistry, 22.12.2021 14:00

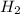 = 0.800 mole
= 0.800 mole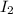 = 0.800 mole
= 0.800 mole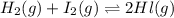
![K_c=\frac{[HI]^2}{[H_2]\times [l_2]}](/tpl/images/0543/3494/7dfaa.png)
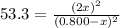
 at equilibrium = 2 x =
at equilibrium = 2 x =