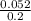Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3


Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
You know the right answer?
What is the numerical value of Kc for the following reaction if the equilibrium mixture at 750 °C co...
Questions

Chemistry, 16.07.2019 13:50



Mathematics, 16.07.2019 13:50

History, 16.07.2019 13:50



Biology, 16.07.2019 13:50


Mathematics, 16.07.2019 13:50




Mathematics, 16.07.2019 13:50

History, 16.07.2019 13:50

Chemistry, 16.07.2019 13:50

Mathematics, 16.07.2019 13:50



Mathematics, 16.07.2019 13:50

![\frac{[CO_{2}]}{[CO]}](/tpl/images/0543/2562/ade69.png)