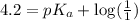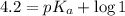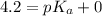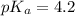Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 09:30
Where are the noble gases located in the periodic table? a. in the center b. on the left side c. in the upper right corner d. on the far right side
Answers: 1
You know the right answer?
An unknown acid is titrated with a strong base. At the half-equivalence point (i. e., at the volume...
Questions


Business, 27.02.2021 07:30



Mathematics, 27.02.2021 07:30

Biology, 27.02.2021 07:30


Social Studies, 27.02.2021 07:30


Spanish, 27.02.2021 07:30

English, 27.02.2021 07:30


Arts, 27.02.2021 07:30


English, 27.02.2021 07:30


Physics, 27.02.2021 07:40



Mathematics, 27.02.2021 07:40

![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0543/2312/e961a.png)