
Chemistry, 11.03.2020 21:14 kolbehoneyman
An old antacid commercial claimed that each tablet of their product could neutralize 47 times its mass in stomach acid. The active ingredient in the antacid tablet, NaAl ( OH ) 2 CO 3 , NaAl(OH)2CO3, reacts with the HCl HCl in stomach acid according to the balanced reaction here. NaAl ( OH ) 2 CO 3 + 4 HCl ⟶ NaCl + AlCl 3 + 3 H 2 O + CO 2 NaAl(OH)2CO3+4HCl⟶NaCl+AlCl3+3H2O+C O2 How many moles of HCl HCl can a 1.24 1.24 g antacid tablet neutralize if the tablet contains 0.296 0.296 g of the active ingredient?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
An old antacid commercial claimed that each tablet of their product could neutralize 47 times its ma...
Questions






Computers and Technology, 15.07.2020 02:01



Computers and Technology, 15.07.2020 02:01











Mathematics, 15.07.2020 02:01

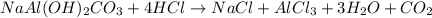
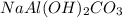
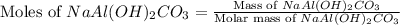
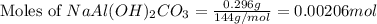
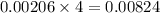 moles of HCl
moles of HCl

