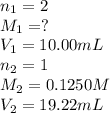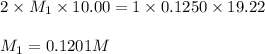Chemistry, 11.03.2020 18:35 genyjoannerubiera
You are using a standardized 0.1250 M solution of sodium hydroxide to determine the concentration of sulfuric acid. After pipetting 10.00 mL of the H2SO4 into your flask you find that you use 19.22 mL of your NaOH solution to reach the endpoint. No, wait... I mean What is the concentration of the sulfuric acid? H2SO4 + 2NaOH ---> Na2SO4 + 2HOH

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
You are using a standardized 0.1250 M solution of sodium hydroxide to determine the concentration of...
Questions




Mathematics, 04.05.2021 18:40

Computers and Technology, 04.05.2021 18:40



Computers and Technology, 04.05.2021 18:40

English, 04.05.2021 18:40

Mathematics, 04.05.2021 18:40

Mathematics, 04.05.2021 18:40


Computers and Technology, 04.05.2021 18:40




World Languages, 04.05.2021 18:40



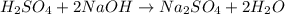
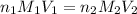
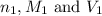 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 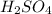
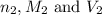 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.