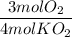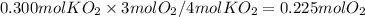The reaction between potassium superoxide, KO₂, and CO₂,
is used as a source of O₂ and...

Chemistry, 11.03.2020 17:25 gadgetady5699
The reaction between potassium superoxide, KO₂, and CO₂,
is used as a source of O₂ and absorber of CO₂ in self-contained breathing equipment used by rescue workers.
How many moles of O₂ are produced when 0.300 mol of KO₂ reacts in this fashion?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1


Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
Questions


Geography, 16.12.2020 21:10

Biology, 16.12.2020 21:10

Arts, 16.12.2020 21:10



Mathematics, 16.12.2020 21:10



Mathematics, 16.12.2020 21:10

Mathematics, 16.12.2020 21:10

History, 16.12.2020 21:10




Mathematics, 16.12.2020 21:10

Social Studies, 16.12.2020 21:10

Mathematics, 16.12.2020 21:10

Biology, 16.12.2020 21:10

Mathematics, 16.12.2020 21:10