
Chemistry, 11.03.2020 06:06 QuestionsAnsweredNow
A mixture of CO2 and Kr weighs 41.0 g and exerts a pressure of 0.729 atm in its container. Since Kr is expensive, you wish to recover it from the mixture. After the CO2 is completely removed by absorption with NaOH(s), the pressure in the container is 0.193 atm. (a) How many grams of CO2 were originally present? (b) How many grams of Kr can you recover?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1


Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

You know the right answer?
A mixture of CO2 and Kr weighs 41.0 g and exerts a pressure of 0.729 atm in its container. Since Kr...
Questions




Mathematics, 20.04.2021 00:44

Social Studies, 20.04.2021 00:44


History, 20.04.2021 00:44





Physics, 20.04.2021 00:44

English, 20.04.2021 00:44

Mathematics, 20.04.2021 00:44

Biology, 20.04.2021 00:44


Mathematics, 20.04.2021 00:44


Mathematics, 20.04.2021 00:44

Mathematics, 20.04.2021 00:44

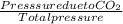
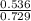
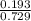
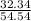 * 100 %
* 100 % 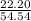 * 100 %
* 100 % 

