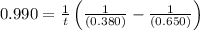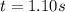Chemistry, 11.03.2020 06:13 dianacastro8298
The rate constant for this second‑order reaction is 0.990 M − 1 ⋅ s − 1 0.990 M−1⋅s−1 at 300 ∘ C. 300 ∘C. A ⟶ products A⟶products How long, in seconds, would it take for the concentration of A A to decrease from 0.650 M 0.650 M to 0.380 M?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2


Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1
You know the right answer?
The rate constant for this second‑order reaction is 0.990 M − 1 ⋅ s − 1 0.990 M−1⋅s−1 at 300 ∘ C. 30...
Questions

Mathematics, 19.11.2020 22:20

Mathematics, 19.11.2020 22:20

Physics, 19.11.2020 22:20


Mathematics, 19.11.2020 22:20


Mathematics, 19.11.2020 22:20


Mathematics, 19.11.2020 22:20



Chemistry, 19.11.2020 22:20






Mathematics, 19.11.2020 22:20


![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0542/6727/5ea71.png)
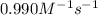
![[A]_o](/tpl/images/0542/6727/9caf5.png) = Initial concentration = 0.650 M
= Initial concentration = 0.650 M