
Chemistry, 11.03.2020 04:33 ashleyjohnson2002
(a) Write the dissolution reaction for solid Na2CO3 below. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.)
(b) Once the ionic solid has dissolved, the anion that is formed is able to react as a base, with water as the acid. Write the net acid-base reaction that occurs when dissolved Na2CO3 reacts with water. (Use the lowest possible coefficients. Omit states-of-matter in your answer.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

You know the right answer?
(a) Write the dissolution reaction for solid Na2CO3 below. (Use the lowest possible coefficients. In...
Questions


Biology, 12.09.2019 20:30



Biology, 12.09.2019 20:30





Biology, 12.09.2019 20:30


Biology, 12.09.2019 20:30


Biology, 12.09.2019 20:30



Biology, 12.09.2019 20:30



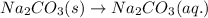
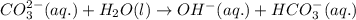
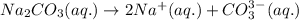
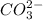 reacts with water to form conjugate acid.
reacts with water to form conjugate acid.


