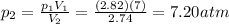Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1

Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3
You know the right answer?
A gas has a pressure of 2.82 atm and occupies a volume of 7 L. If the gas is compressed to a volume...
Questions


Physics, 17.10.2019 08:30

Physics, 17.10.2019 08:30



Geography, 17.10.2019 08:30



Biology, 17.10.2019 08:30


History, 17.10.2019 08:30


Mathematics, 17.10.2019 08:30

Mathematics, 17.10.2019 08:30


Mathematics, 17.10.2019 08:30



Biology, 17.10.2019 08:30

Mathematics, 17.10.2019 08:30

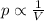
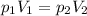
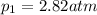 is the initial pressure of the gas
is the initial pressure of the gas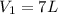 is the initial volume of the gas
is the initial volume of the gas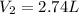 is the final volume of the gas
is the final volume of the gas