
The following equation shows the equilibrium in an aqueous solution of ammonia: NH3(aq)+H2O(l)⇌NH4+(aq)+OH−(aq)NH3( aq)+H2O(l)⇌NH4+(aq)+OH−(aq) Which of the following represents a conjugate acid-base pair?a) NH3 and H2O b) NH4+ and OH− c) H2O and OH− d) NH3 and OH−

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
You know the right answer?
The following equation shows the equilibrium in an aqueous solution of ammonia: NH3(aq)+H2O(l)⇌NH4+(...
Questions

Mathematics, 21.07.2019 05:00






Social Studies, 21.07.2019 05:00



Social Studies, 21.07.2019 05:00








Mathematics, 21.07.2019 05:00

History, 21.07.2019 05:00

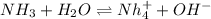
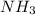 and the conjugate acid of the base is
and the conjugate acid of the base is  .
. and the conjugate acid of the base is
and the conjugate acid of the base is 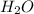 .
.

