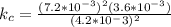A sample of pure NO2NO2 is heated to 335 ∘C∘C at which temperature it partially dissociates according to the equation 2NO2(g)⇌2NO(g)+O2(g)2NO2(g)⇌2NO(g)+ O2(g) At equilibrium the density of the gas mixture is 0.525 g/Lg/L at 0.750 atmatm .Calculate Kc for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1


Chemistry, 23.06.2019 11:00
Asolubility table shows that almost all compounds of group 1 metals are soluble in water. this general rule tells you that mgi2 is soluble rbno3 is soluble cacl2 is soluble co2 is soluble
Answers: 1

Chemistry, 23.06.2019 17:00
An unknown substance was put in a container. the substance filled up the container from bottom up, taking half of the space in the container. what is most likely the state of matter of the substance? gas liquid plasma solid
Answers: 3
You know the right answer?
A sample of pure NO2NO2 is heated to 335 ∘C∘C at which temperature it partially dissociates accordin...
Questions

Health, 04.11.2020 22:00




History, 04.11.2020 22:00





History, 04.11.2020 22:00




Mathematics, 04.11.2020 22:00



Health, 04.11.2020 22:00



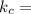 1. 1 × 10⁻²
1. 1 × 10⁻²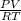
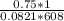
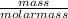
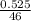
![k_c = \frac{[NO]^2[O_2]}{[NO_2]^2}](/tpl/images/0542/3911/d520a.png)