Chemistry, 11.03.2020 02:34 KillerSteamcar
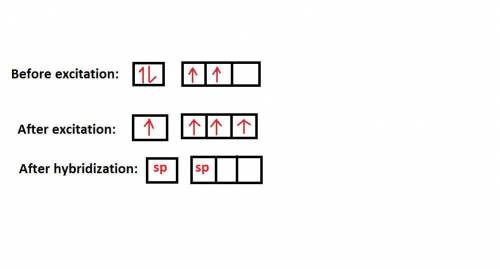
Consider a carbon atom that is sp hybridized. Indicate how many of each orbital exist on this carbon atom by sorting each orbital type. Consider the outer valence only.
Options include: sp orbitals, p orbitals, s orbitals
Put them in the following categories:
Zero One Two Three

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Consider a carbon atom that is sp hybridized. Indicate how many of each orbital exist on this carbon...
Questions

Mathematics, 02.04.2020 21:10




Spanish, 02.04.2020 21:10




Chemistry, 02.04.2020 21:10

History, 02.04.2020 21:10



Mathematics, 02.04.2020 21:11



Law, 02.04.2020 21:11

Mathematics, 02.04.2020 21:11



Mathematics, 02.04.2020 21:11

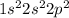 . This means that in its neutral state it contains 2 electrons in its s-orbital and 2 electrons in its p-orbital.
. This means that in its neutral state it contains 2 electrons in its s-orbital and 2 electrons in its p-orbital.