
Chemistry, 11.03.2020 02:25 unicornpoop54
A certain chemical reaction releases 480. kJ of heat energy per mole of reactant consumed. Suppose some moles of the reactant are put into a calorimeter (a device for measuring heat flow). It takes 3.85 J of heat energy to raise the temperature of this calorimeter by 1 °C. Now the reaction is run until all the reactant is gone, and the temperature of the calorimeter is found to rise by 14.6 °C. How would you calculate the number of moles of reactant that were consumed? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. moles consumed-

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
A certain chemical reaction releases 480. kJ of heat energy per mole of reactant consumed. Suppose s...
Questions

English, 30.06.2019 01:30


Computers and Technology, 30.06.2019 01:30

Biology, 30.06.2019 01:30



Mathematics, 30.06.2019 01:30



History, 30.06.2019 01:30

Biology, 30.06.2019 01:30

Geography, 30.06.2019 01:30

Social Studies, 30.06.2019 01:30


Biology, 30.06.2019 01:30

English, 30.06.2019 01:30

Mathematics, 30.06.2019 01:30



Mathematics, 30.06.2019 01:30

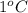 . So here, specific heat is given as 3.85 J. And, the change in temperature is
. So here, specific heat is given as 3.85 J. And, the change in temperature is 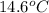 .
.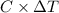
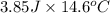
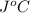
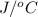 of heat is calculated as follows.
of heat is calculated as follows.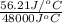
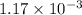 moles
moles

