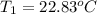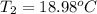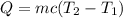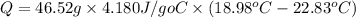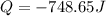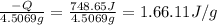Chemistry, 11.03.2020 02:11 zacharycheyne
The heat of solution is found by adding a salt to water in a calorimeter and measuring the temperature change. The specific heat of water is 4.180 Joules per g per ºC. In the calculation of the heat of solution, ignore the contribution to specific heat and mass due to the salt. Assume that these contributions are negligible. The data collected are as follows:
Grams of water in the calorimeter 46.52
Grams of salt 4.5069
Initial temperature of water 22.83 ºC
Final Temperature 18.98 ºC
Calculate the following Heat of the solution of salt.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1
You know the right answer?
The heat of solution is found by adding a salt to water in a calorimeter and measuring the temperatu...
Questions

Mathematics, 27.08.2021 01:00


Advanced Placement (AP), 27.08.2021 01:00

Social Studies, 27.08.2021 01:00

Mathematics, 27.08.2021 01:00

Social Studies, 27.08.2021 01:00


Mathematics, 27.08.2021 01:00

Biology, 27.08.2021 01:00

Mathematics, 27.08.2021 01:00



Health, 27.08.2021 01:00




Chemistry, 27.08.2021 01:00

Physics, 27.08.2021 01:00

Mathematics, 27.08.2021 01:00