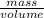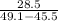Chemistry, 10.03.2020 23:07 hgdthbgjnb5604
A 28.5 gram piece of iron is added to a graduated cylinder containing 45.5 mL of water. The water in the cylinder rises to the 49.1 mark. Calculate the density of the iron piece.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1
You know the right answer?
A 28.5 gram piece of iron is added to a graduated cylinder containing 45.5 mL of water. The water in...
Questions

Arts, 18.01.2021 01:50

Mathematics, 18.01.2021 01:50



Biology, 18.01.2021 01:50

Mathematics, 18.01.2021 01:50



History, 18.01.2021 01:50

Mathematics, 18.01.2021 01:50

Mathematics, 18.01.2021 01:50

SAT, 18.01.2021 01:50

History, 18.01.2021 01:50




Mathematics, 18.01.2021 01:50