
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. H+ + H2O2 ? H3O2+ (rapid equilibrium) H3O2+ + Br- ? HOBr + H2O (slow) HOBr+H+ +Br- ?Br2 +H2O(fast) Which rate law is consistent with this mechanism?
a. k[Br-][H+]-1[H2O2]-1
b. k[H+][H2O2][Br-]
c. k[H+][H2O2]
d. k[HOBr][H+][Br-]

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous a...
Questions

History, 27.09.2019 18:00








English, 27.09.2019 18:00




Mathematics, 27.09.2019 18:00

Mathematics, 27.09.2019 18:00



English, 27.09.2019 18:00



![\text{Rate}=k'[H+][H_2O_2][Br^-]](/tpl/images/0541/5354/e35f3.png)
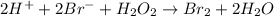
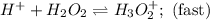
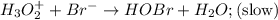
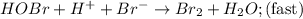
![\text{Rate}=k[H_3O_2^+][Br^-]](/tpl/images/0541/5354/37d2f.png) ......(1)
......(1)![[H_3O_2^+]](/tpl/images/0541/5354/85c88.png) is not appearing as a reactant in the overall reaction. So, we apply steady state approximation in it.
is not appearing as a reactant in the overall reaction. So, we apply steady state approximation in it.![K=\frac{[H_3O_2^+]}{[H^+][H_2O_2]}](/tpl/images/0541/5354/4cd3a.png)
![[H_3O_2^+]=K[H^+][H_2O_2]](/tpl/images/0541/5354/15cd9.png)
![\text{Rate}=k.K[H^+][H_2O_2][Br^-]\\\\\text{Rate}=k'[H+][H_2O_2][Br^-]](/tpl/images/0541/5354/f918c.png)


