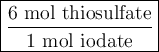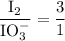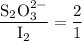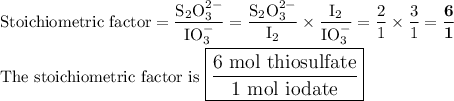Part III. The two reactions involved in quantitatively determining the amount of iodate in solution are: IO3-(aq) 5 I-(aq) 6 H (aq) --> 3 I2(aq) 3 H2O(l) followed by reaction of the I2: I2(aq) 2 S2O32- --> 2 I-(aq) S4O62-(aq). What is the stoichiometric factor, that is the number of moles of Na2S2O3 reacting with one mole of KIO3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1
You know the right answer?
Part III. The two reactions involved in quantitatively determining the amount of iodate in solution...
Questions

Mathematics, 25.11.2020 05:40

Health, 25.11.2020 05:40


Mathematics, 25.11.2020 05:40








Mathematics, 25.11.2020 05:40


History, 25.11.2020 05:40

Mathematics, 25.11.2020 05:40



Computers and Technology, 25.11.2020 05:40