
At a certain temperature the rate of this reaction is first order in HI with a rate constant of :7.21s?1
2HI(g)??H2(g) + I2(g)
Suppose a vessel contains HI at a concentration of 0.440M
. Calculate the concentration of HI in the vessel 0.210 seconds later. You may assume no other reaction is important.
Round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3
You know the right answer?
At a certain temperature the rate of this reaction is first order in HI with a rate constant of :7.2...
Questions

English, 18.03.2021 03:00


Mathematics, 18.03.2021 03:00



Mathematics, 18.03.2021 03:00



Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00






Mathematics, 18.03.2021 03:00

Mathematics, 18.03.2021 03:00


Geography, 18.03.2021 03:00

![[A_o]=0.440 M](/tpl/images/0541/4407/bdc04.png)
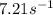
![[A]=[A_o]\times e^{-kt}](/tpl/images/0541/4407/abdec.png)
![[A]=0.440 M\times e^{-7.21 s^{-1}\times 0.210 s}](/tpl/images/0541/4407/4243c.png)


