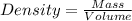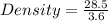Chemistry, 10.03.2020 19:21 gwendallinesikes
A 28.5 gram piece of iron is added to a graduated cylinder containing 45.5 mL of water. The water in the cylinder rises to the 49.1 mark. Calculate the density of the iron piece.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
A 28.5 gram piece of iron is added to a graduated cylinder containing 45.5 mL of water. The water in...
Questions


Mathematics, 29.03.2021 19:00

Social Studies, 29.03.2021 19:00

World Languages, 29.03.2021 19:00

Health, 29.03.2021 19:00


Mathematics, 29.03.2021 19:00

Mathematics, 29.03.2021 19:00

Advanced Placement (AP), 29.03.2021 19:00



Mathematics, 29.03.2021 19:00

Biology, 29.03.2021 19:00

Mathematics, 29.03.2021 19:00

Mathematics, 29.03.2021 19:00

English, 29.03.2021 19:10


Mathematics, 29.03.2021 19:10