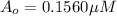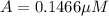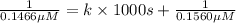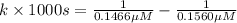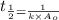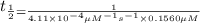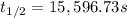Chemistry, 10.03.2020 19:03 leannaadrian
Nitrous acid (HNO2) slowly decomposes to NO, NO2, and water by the following second-order reaction: 2 HNO2(aq) ---> NO(g) + NO2(g) + H2O(l) Use the date below to determine the rate law and the constant for this reaction: Time (s) [HNO2] (um) 0 0.1560 1000 0.1466 1500 0.1424 2000 0.1383 2500 0.1345 3000 0.1309 A) Rate = ?? B) k = ??? /uM*s C) Determine the half-life for the decomposition of HNO2: t1/2 = ?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
Nitrous acid (HNO2) slowly decomposes to NO, NO2, and water by the following second-order reaction:...
Questions




English, 20.11.2020 21:20


Mathematics, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20

English, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20

Arts, 20.11.2020 21:20




English, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20



Chemistry, 20.11.2020 21:20

![R=k[HNO_2]^2](/tpl/images/0541/2623/c6302.png)
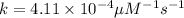
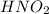 is 15,596.73 s
is 15,596.73 s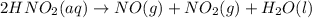
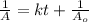
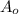 = initial concentration
= initial concentration