

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
Reaction rate is expressed in terms of changes in the concentration of reactants and products. Write...
Questions

Mathematics, 24.09.2020 09:01


Mathematics, 24.09.2020 09:01



Mathematics, 24.09.2020 09:01

Mathematics, 24.09.2020 09:01



English, 24.09.2020 09:01

Mathematics, 24.09.2020 09:01

Mathematics, 24.09.2020 09:01



Mathematics, 24.09.2020 09:01

Mathematics, 24.09.2020 09:01


Mathematics, 24.09.2020 09:01

History, 24.09.2020 09:01

Biology, 24.09.2020 09:01

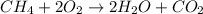
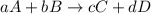
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0541/3019/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0541/3019/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0541/3019/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0541/3019/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0541/3019/d4b94.png)
![Rate=-\frac{d[CH_4]}{dt}=-\frac{1}{2}\frac{d[O_2]}{dt}=+\frac{1}{2}\frac{d[H_2O]}{dt}=+\frac{d[CO_2]}{dt}](/tpl/images/0541/3019/dfd60.png)


