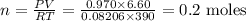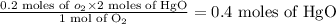An unknown amount of mercury (II) oxide was decomposed in the lab. Mercury metal
was formed an...

Chemistry, 10.03.2020 18:38 sahaitong1844
An unknown amount of mercury (II) oxide was decomposed in the lab. Mercury metal
was formed and 6.60 L of oxygen gas was released at a pressure of 0.970 atm and
390.0 K. What was the initial weight of mercury oxide in the sample? (1 point)
1) 48.91 grams
2) 64.32 grams
3) 78.32 grams
4) 86.6 grams

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Questions

English, 06.07.2019 14:00

Mathematics, 06.07.2019 14:00

Arts, 06.07.2019 14:00


Biology, 06.07.2019 14:00




Mathematics, 06.07.2019 14:00





Mathematics, 06.07.2019 14:00




Computers and Technology, 06.07.2019 14:00


History, 06.07.2019 14:00