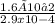Chemistry, 10.03.2020 17:11 sciencecreation87
In an analysis of the following reaction at a certain temperature, Br2(g) + Cl2(g) ⇌ 2BrCl(g)the equilibrium concentrations were found to be [Br2] = 4.5 ×10−3 M, [Cl2] = 2.6 ×10−2 M, and [BrCl] = 1.6 ×10−2 M. Write the equilibrium expression, and calculate the equilibrium constant for this reaction at this temperature.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
In an analysis of the following reaction at a certain temperature, Br2(g) + Cl2(g) ⇌ 2BrCl(g)the equ...
Questions

Mathematics, 01.04.2021 03:30


Mathematics, 01.04.2021 03:30

Mathematics, 01.04.2021 03:30

Mathematics, 01.04.2021 03:30




Physics, 01.04.2021 03:30


Mathematics, 01.04.2021 03:40







English, 01.04.2021 03:40


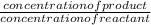
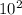
![[BrCl]^{2}](/tpl/images/0541/0381/fca7c.png)
![\frac{[BrCl[2}{[Br]2[Cl]2}](/tpl/images/0541/0381/5dca4.png) equation 1
equation 1![\frac{[1.6 ×10−2]}{[0.45 x 10^-2] [1.6 ×10−2]}](/tpl/images/0541/0381/7a675.png)