Chemistry, 10.03.2020 16:59 adhanom1271
A chemist prepares a solution of potassium permanganate (KMnO 4)by measuring out 4.6 mu mol of potassium permanganate into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmol/L of the chemist's potassium permanganate solution. Round your answer to 2significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
A chemist prepares a solution of potassium permanganate (KMnO 4)by measuring out 4.6 mu mol of potas...
Questions

English, 07.02.2021 01:00

Computers and Technology, 07.02.2021 01:00

Mathematics, 07.02.2021 01:00

Chemistry, 07.02.2021 01:00

Mathematics, 07.02.2021 01:00

Physics, 07.02.2021 01:00



Advanced Placement (AP), 07.02.2021 01:00



Advanced Placement (AP), 07.02.2021 01:00

Computers and Technology, 07.02.2021 01:00


History, 07.02.2021 01:00


Mathematics, 07.02.2021 01:00


Mathematics, 07.02.2021 01:00

Mathematics, 07.02.2021 01:00

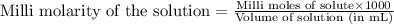
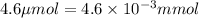 (Conversion factor:
(Conversion factor: