
Chemistry, 10.03.2020 09:01 devin030505
Problem PageQuestion Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 8.42 g of ethane is mixed with 48. g of oxygen. Calculate the minimum mass of ethane that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3


Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1
You know the right answer?
Problem PageQuestion Gaseous ethane will react with gaseous oxygen to produce gaseous carbon dioxide...
Questions


English, 01.04.2021 23:00

Biology, 01.04.2021 23:00



History, 01.04.2021 23:00

Chemistry, 01.04.2021 23:00





Mathematics, 01.04.2021 23:00

Mathematics, 01.04.2021 23:00

Mathematics, 01.04.2021 23:00




Spanish, 01.04.2021 23:00


Mathematics, 01.04.2021 23:00

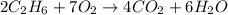
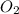 react completely with 2 moles of
react completely with 2 moles of 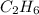
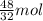 of
of 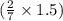 moles of
moles of 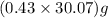 = 12.9 g
= 12.9 g

