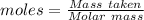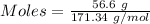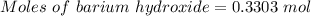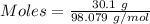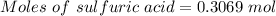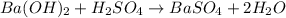For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of sulfuric acid. barium hydroxide (aq) + sulfuric acid (aq) barium sulfate (s) + water (l) What is the maximum amount of barium sulfate that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of s...
Questions

History, 08.03.2021 04:20

Health, 08.03.2021 04:20

Mathematics, 08.03.2021 04:20


Biology, 08.03.2021 04:20

Spanish, 08.03.2021 04:20

Mathematics, 08.03.2021 04:20



Mathematics, 08.03.2021 04:20

Mathematics, 08.03.2021 04:20






Advanced Placement (AP), 08.03.2021 04:20

Social Studies, 08.03.2021 04:20


Arts, 08.03.2021 04:20

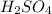 .
.