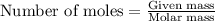Chemistry, 10.03.2020 09:04 mostman077
A titration of vinegar with a solution of NaOH was performed. If 3.45 mL of vinegar needs 44.0 mL of 0.140 M NaOH to reach the equivalence point in a titration, Calculate the mass of acetic acid present in the vinegar sample: mastering chemistry answers

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
A titration of vinegar with a solution of NaOH was performed. If 3.45 mL of vinegar needs 44.0 mL of...
Questions

History, 20.08.2019 22:40

Mathematics, 20.08.2019 22:40

Mathematics, 20.08.2019 22:40



Mathematics, 20.08.2019 22:40


Mathematics, 20.08.2019 22:40

Mathematics, 20.08.2019 22:40




Biology, 20.08.2019 22:40

History, 20.08.2019 22:40

History, 20.08.2019 22:40

Geography, 20.08.2019 22:40

Mathematics, 20.08.2019 22:40

History, 20.08.2019 22:40


Mathematics, 20.08.2019 22:40

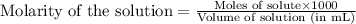
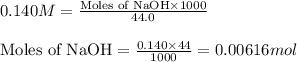
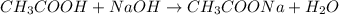
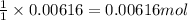 of acetic acid
of acetic acid