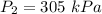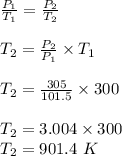Chemistry, 10.03.2020 09:01 alexis3060
A 3.0 L container holds a sample of hydrogen gas at 300 K and 101.5 kPa . The pressure increases to 305 kPa and the volume remains constant. What will the temperature be?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
A 3.0 L container holds a sample of hydrogen gas at 300 K and 101.5 kPa . The pressure increases to...
Questions

English, 30.11.2021 18:10

English, 30.11.2021 18:10

Mathematics, 30.11.2021 18:10




Mathematics, 30.11.2021 18:10

Advanced Placement (AP), 30.11.2021 18:10



Mathematics, 30.11.2021 18:10


History, 30.11.2021 18:10

Mathematics, 30.11.2021 18:10

Mathematics, 30.11.2021 18:10





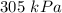 is
is 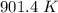 .
.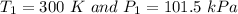
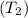 of the gas at
of the gas at