

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
If 61.1 mL of lead(II) nitrate solution reacts completely with excess sodium iodide solution to yiel...
Questions


Biology, 02.02.2020 14:45

Mathematics, 02.02.2020 14:45



Mathematics, 02.02.2020 14:45

English, 02.02.2020 14:45

History, 02.02.2020 14:45


Social Studies, 02.02.2020 14:45

Biology, 02.02.2020 14:45

Social Studies, 02.02.2020 14:45





Biology, 02.02.2020 14:45

Computers and Technology, 02.02.2020 14:46


Chemistry, 02.02.2020 14:46

 is precipitated as
is precipitated as  when NaI is added to solution of
when NaI is added to solution of 
 mol of
mol of 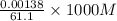 = 0.0226 M
= 0.0226 M

