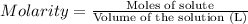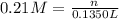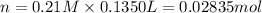Chemistry, 10.03.2020 08:58 jdodger5165
A chemist adds 135.0 mL of a 0.21M zinc nitrate (Zn(NO3) solution to a reaction flask. Calculate the mass in grams of zinc nitrate the chemist has added to the flask. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
A chemist adds 135.0 mL of a 0.21M zinc nitrate (Zn(NO3) solution to a reaction flask. Calculate the...
Questions

Physics, 18.10.2019 04:00




Mathematics, 18.10.2019 04:00



Mathematics, 18.10.2019 04:00

Mathematics, 18.10.2019 04:00


English, 18.10.2019 04:00


Business, 18.10.2019 04:00


Health, 18.10.2019 04:00

Social Studies, 18.10.2019 04:00

Mathematics, 18.10.2019 04:00