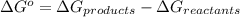For the following reaction, label each of the below species as an acid or a base. Use lower case letters only (e. g. acid)
HCN + HPO4⁻² ⇔ H2PO4⁻ + CN⁻
Will the above reaction take place spontaneously? (Is the reaction product-favored? Does the equilibrium lie to the right?)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
For the following reaction, label each of the below species as an acid or a base. Use lower case let...
Questions


Computers and Technology, 29.09.2020 14:01


Spanish, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01


Health, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01


Geography, 29.09.2020 14:01


Mathematics, 29.09.2020 14:01


History, 29.09.2020 14:01


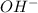 .
. 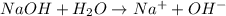
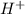 .
.
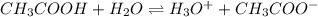
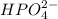 is accepting the hydrogen ions so it acts as a base.
is accepting the hydrogen ions so it acts as a base.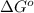 values of the given species are as follows.
values of the given species are as follows.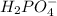 = -1130.4 kJ/mol,
= -1130.4 kJ/mol, 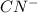 = 172.4 kJ/mol
= 172.4 kJ/mol