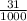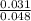Chemistry, 10.03.2020 08:16 jordandabrat
1) You have an aqueous solution where [OH-] = 1 x 10-4 mol/L.
A. Calculate the hydrogen ion concentration [H+]. Show all work.
B. Calculate the pH. Show all work.
C. Is this solution an acid, base, or neutral? You must explain how you know.
2) Calculate the molarity of a solution prepared by dissolving 195 g of sodium chloride (NaCl) in enough water to make 2.8 liters of solution.
3) A student takes 152 mL of a 3.0M HCl solution and places it into a beaker. The student then adds enough water to the beaker to make 750 mL. Calculate the molarity of this new solution. Show all work.
4) A student started a titration by placing 20.0 mL of 0.24 M NaOH into a flask with 3 drops of phenolphthalein. The student then titrated to the endpoint using 31.0 mL of HCl. Calculate the concentration (molarity) of the HCl that the student used. Show all work for credit.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1


Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2
You know the right answer?
1) You have an aqueous solution where [OH-] = 1 x 10-4 mol/L.
A. Calculate the hydrogen ion co...
A. Calculate the hydrogen ion co...
Questions







Mathematics, 21.03.2020 23:00


History, 21.03.2020 23:00

Social Studies, 21.03.2020 23:00

World Languages, 21.03.2020 23:00

Mathematics, 21.03.2020 23:00


Mathematics, 21.03.2020 23:00

Social Studies, 21.03.2020 23:00


Geography, 21.03.2020 23:00

Mathematics, 21.03.2020 23:00


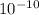 x is the hydrogen ion concentration.
x is the hydrogen ion concentration.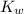 = [
= [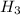 O+] [OH]-
O+] [OH]- 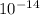
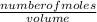
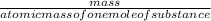
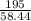 ( atomic weight of NaCl is 58.44 gm/mole)
( atomic weight of NaCl is 58.44 gm/mole)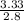
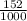 = M2 ×
= M2 × 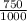
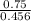
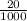 = Macid x
= Macid x