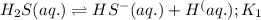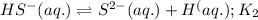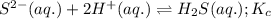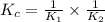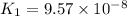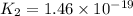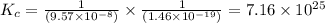Chemistry, 10.03.2020 07:49 TheCampingStone
Given the two reactions H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.57×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.46×10−19, what is the equilibrium constant Kfinal for the following reaction? S2−(aq)+2H+(aq)⇌H2S(aq) Enter your answer numerically.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1

Chemistry, 23.06.2019 10:30
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1

Chemistry, 23.06.2019 17:30
How do you determine the degree of a power function from a table? when do you know you have successfully determined the degree of the power function?
Answers: 1
You know the right answer?
Given the two reactions H2S(aq)⇌HS−(aq)+H+(aq), K1 = 9.57×10−8, and HS−(aq)⇌S2−(aq)+H+(aq), K2 = 1.4...
Questions



Biology, 13.06.2021 01:00




Mathematics, 13.06.2021 01:00

Mathematics, 13.06.2021 01:00

Mathematics, 13.06.2021 01:00


Mathematics, 13.06.2021 01:00

Mathematics, 13.06.2021 01:00








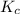 for the final reaction is
for the final reaction is