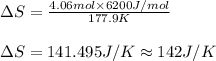The heat of fusion of dichloromethane (CH2Cl2) is 6.2 kJ/mol.
Calculate the change in entropy...

Chemistry, 10.03.2020 07:28 umimgoingtofail
The heat of fusion of dichloromethane (CH2Cl2) is 6.2 kJ/mol.
Calculate the change in entropy Δs when 345 g of dichloromethane freezes at -95.1 °C.
Be sure your answer contains a unit symbol and the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Questions

Business, 09.12.2019 07:31

Mathematics, 09.12.2019 07:31


Mathematics, 09.12.2019 07:31

History, 09.12.2019 07:31

Computers and Technology, 09.12.2019 07:31

Mathematics, 09.12.2019 07:31

Social Studies, 09.12.2019 07:31


Geography, 09.12.2019 07:31

Mathematics, 09.12.2019 07:31

Mathematics, 09.12.2019 07:31

Mathematics, 09.12.2019 07:31

Mathematics, 09.12.2019 07:31

Mathematics, 09.12.2019 07:31

Mathematics, 09.12.2019 07:31

English, 09.12.2019 07:31

Mathematics, 09.12.2019 07:31


Arts, 09.12.2019 07:31

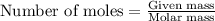
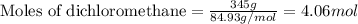
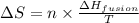
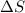 = Entropy change = ?
= Entropy change = ?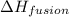 = enthalpy of fusion = 6.2 kJ/mol = 6200 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of fusion = 6.2 kJ/mol = 6200 J/mol (Conversion factor: 1 kJ = 1000 J)![-95.1^oC=[-95.1+273]K=177.9K](/tpl/images/0540/3534/1b16d.png)