Chemistry, 10.03.2020 07:25 hhvgbv49551
The heat of fusion ΔHf of toluene C6H5CH3 is 6.6 /kJmol . Calculate the change in entropy ΔS when 46.g of toluene melts at −95.0°C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3


Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
The heat of fusion ΔHf of toluene C6H5CH3 is 6.6 /kJmol . Calculate the change in entropy ΔS when 46...
Questions


History, 19.11.2019 09:31

History, 19.11.2019 09:31

English, 19.11.2019 09:31



Biology, 19.11.2019 09:31


Mathematics, 19.11.2019 09:31

English, 19.11.2019 09:31

Chemistry, 19.11.2019 09:31

Chemistry, 19.11.2019 09:31

Mathematics, 19.11.2019 09:31

Chemistry, 19.11.2019 09:31

Mathematics, 19.11.2019 09:31



English, 19.11.2019 09:31


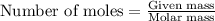
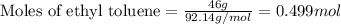
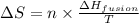
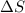 = Entropy change = ?
= Entropy change = ?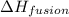 = enthalpy of fusion = 6.6 kJ/mol = 6600 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of fusion = 6.6 kJ/mol = 6600 J/mol (Conversion factor: 1 kJ = 1000 J)![-95.0^oC=[-95.0+273]K=178K](/tpl/images/0540/3339/0d1c2.png)