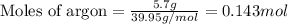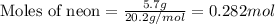Chemistry, 10.03.2020 07:22 lololol270
A gas mixture is made by combining 5.7 g each of Ar , Ne , and an unknown diatomic gas. At STP, the mixture occupies a volume of 12.88 L. What is the molar mass of the unknown gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

You know the right answer?
A gas mixture is made by combining 5.7 g each of Ar , Ne , and an unknown diatomic gas. At STP, the...
Questions


Mathematics, 03.09.2021 09:20


Mathematics, 03.09.2021 09:20

Mathematics, 03.09.2021 09:20

Mathematics, 03.09.2021 09:20


Mathematics, 03.09.2021 09:20


Mathematics, 03.09.2021 09:20


Geography, 03.09.2021 09:20

Mathematics, 03.09.2021 09:20



Social Studies, 03.09.2021 09:20

Mathematics, 03.09.2021 09:20

Mathematics, 03.09.2021 09:20


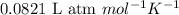
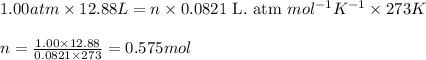
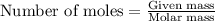 .....(1)
.....(1)