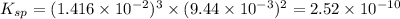Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
1.24 grams of magnesium phosphate tribasic dissolved in 1 L of lemon juice. What is the Ksp of the m...
Questions





Biology, 11.05.2021 07:10




Mathematics, 11.05.2021 07:10

Mathematics, 11.05.2021 07:10

Computers and Technology, 11.05.2021 07:10


Computers and Technology, 11.05.2021 07:10



Mathematics, 11.05.2021 07:10


History, 11.05.2021 07:10


History, 11.05.2021 07:10

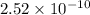
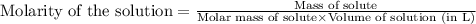
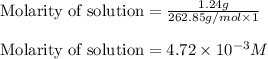
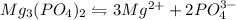
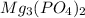 will be:
will be:![K_{sp}=[Mg^{2+}]^3[PO_4^{3-}]^2](/tpl/images/0540/2821/707f8.png)
![[Mg^{2+}]=(3\times 4.72\times 10^{-3})=1.416\times 10^{-2}M](/tpl/images/0540/2821/1537a.png)
![[PO_4^{3-}]=(2\times 4.72\times 10^{-3})=9.44\times 10^{-3}M](/tpl/images/0540/2821/66769.png)