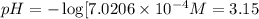Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
Ascorbic acid (H2C6H6O6; H2Asc for this problem), known as vitamin C, is a diprotic acid (Ka1= 1.0x1...
Questions

Mathematics, 08.04.2021 02:40

History, 08.04.2021 02:40

Mathematics, 08.04.2021 02:40


Mathematics, 08.04.2021 02:40

Mathematics, 08.04.2021 02:40


Mathematics, 08.04.2021 02:40


Biology, 08.04.2021 02:40


Mathematics, 08.04.2021 02:50

Chemistry, 08.04.2021 02:50

Social Studies, 08.04.2021 02:50


Mathematics, 08.04.2021 02:50

Mathematics, 08.04.2021 02:50



![[HAsc^-]=0.000702 M](/tpl/images/0540/1441/1ed4d.png)
![[Asc^{2-}]=5.92\times 10^{-8} M](/tpl/images/0540/1441/2fe40.png)
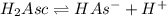
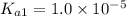
![K_{a1}=\frac{[HAs^-][H^+]}{[H_2Asc]}](/tpl/images/0540/1441/87b37.png)
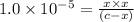
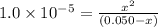
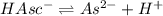
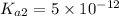
![K_{a2}=\frac{[As^{2-}][H^+]}{[HAsc^-]}](/tpl/images/0540/1441/64e9e.png)
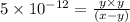
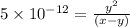
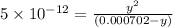
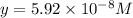
![[H^+]=x+y=0.000702 M+5.92\times 10^{-8} M=7.0206\times 10^{-4} M](/tpl/images/0540/1441/4ae74.png)
![pH=-\log[H^+]](/tpl/images/0540/1441/cf945.png)