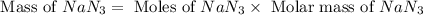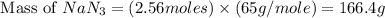The air bags in cars are inflated when a collision triggers the explosive, highly exothermic decomposition of sodium azide (NaN3): 2NaN3(s) → 2Na(s) + 3N2(g) The passenger-side air bag in a typical car must fill a space approximately four times as large as the driver-side bag to be effective. Calculate the mass of sodium azide required to fill a 113-L air bag. Assume the pressure in the car is 1.00 atm and the temperature of N2 produced is 85°C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1
You know the right answer?
The air bags in cars are inflated when a collision triggers the explosive, highly exothermic decompo...
Questions

Biology, 09.07.2019 21:30

Biology, 09.07.2019 21:30

Chemistry, 09.07.2019 21:30

Biology, 09.07.2019 21:30

Mathematics, 09.07.2019 21:30

Chemistry, 09.07.2019 21:30



Biology, 09.07.2019 21:30







Physics, 09.07.2019 21:30



English, 09.07.2019 21:30

Mathematics, 09.07.2019 21:30

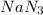 required is, 166.4 grams.
required is, 166.4 grams.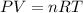
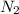 gas = 1.00 atm
gas = 1.00 atm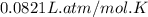
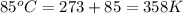
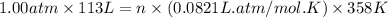
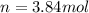
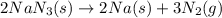
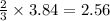 moles of
moles of