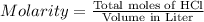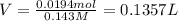Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations would be expected to neutralize. Assume complete neutralization.
a. A tablet containting 350 mg Al(OH)3 and 250 mg Mg(OH)2.
b. A tablet containing 970 mg of CaCO3.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

You know the right answer?
Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations...
Questions

English, 18.02.2021 05:10

Health, 18.02.2021 05:10

History, 18.02.2021 05:10

Mathematics, 18.02.2021 05:10

Mathematics, 18.02.2021 05:10


English, 18.02.2021 05:10

Biology, 18.02.2021 05:10

English, 18.02.2021 05:10

Mathematics, 18.02.2021 05:10

Mathematics, 18.02.2021 05:10

Mathematics, 18.02.2021 05:10

Mathematics, 18.02.2021 05:10

History, 18.02.2021 05:10



Chemistry, 18.02.2021 05:10

Mathematics, 18.02.2021 05:10


Computers and Technology, 18.02.2021 05:10

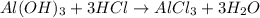
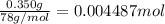
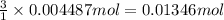 of HCl.
of HCl.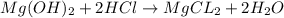
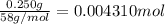
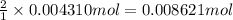 of HCl.
of HCl.
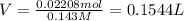
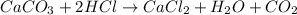
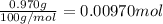
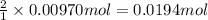 of HCl.
of HCl.