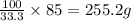Chemistry, 10.03.2020 04:47 morenodonaldo762
A chemistry student needs 85.0g of acetic acid for an experiment. She has available 0.20kg of a 33.3% w/w solution of acetic acid in ethanol. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
A chemistry student needs 85.0g of acetic acid for an experiment. She has available 0.20kg of a 33.3...
Questions

Mathematics, 09.07.2019 19:00


French, 09.07.2019 19:00

Mathematics, 09.07.2019 19:00

French, 09.07.2019 19:00


Arts, 09.07.2019 19:00


Mathematics, 09.07.2019 19:00


Arts, 09.07.2019 19:00


Chemistry, 09.07.2019 19:00


Mathematics, 09.07.2019 19:00

Mathematics, 09.07.2019 19:00


English, 09.07.2019 19:00