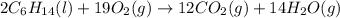Chemistry, 10.03.2020 04:26 spazzinchicago
List the number of each type of atom on the left side of the equation 2C6H14(l)+19O2(g)→12CO2(g)+14H2O(g) Enter your answers separated by commas (the order of the numbers is the same as the order of the elements on the left side of the equation).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
List the number of each type of atom on the left side of the equation 2C6H14(l)+19O2(g)→12CO2(g)+14H...
Questions




Chemistry, 21.02.2020 22:32



Mathematics, 21.02.2020 22:32

English, 21.02.2020 22:32








Mathematics, 21.02.2020 22:33


Computers and Technology, 21.02.2020 22:33

Biology, 21.02.2020 22:33