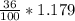Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
What volume of a concentrated HCl solution, which is 36.0% HCl by mass and has a density of 1.179 g/...
Questions

Chemistry, 03.05.2021 23:20

Business, 03.05.2021 23:20


Biology, 03.05.2021 23:20

Social Studies, 03.05.2021 23:20

Mathematics, 03.05.2021 23:20



History, 03.05.2021 23:20

English, 03.05.2021 23:20

Mathematics, 03.05.2021 23:20

History, 03.05.2021 23:20


History, 03.05.2021 23:20

English, 03.05.2021 23:20





Mathematics, 03.05.2021 23:20