
Chemistry, 10.03.2020 04:50 MathChic68
The following reaction was performed in a sealed vessel at 791 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, only H2 and I2 were present at concentrations of [H2]=3.10M and [I2]=2.50M . The equilibrium concentration of I2 is 0.0800 M . What is the equilibrium constant, Kc, for the reaction at this temperature?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1


Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
The following reaction was performed in a sealed vessel at 791 ∘C : H2(g)+I2(g)⇌2HI(g) Initially, on...
Questions

Mathematics, 11.04.2021 20:00



Business, 11.04.2021 20:00


English, 11.04.2021 20:00




Mathematics, 11.04.2021 20:00

Mathematics, 11.04.2021 20:00

Mathematics, 11.04.2021 20:00






History, 11.04.2021 20:00


Biology, 11.04.2021 20:00

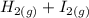 ⇌
⇌ 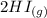
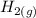

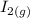 ⇌
⇌ ![K_c = \frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0540/0427/6b81e.png)
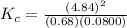
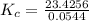
 430.62
430.62 ≅ 431
≅ 431 


