
Chemistry, 10.03.2020 04:11 fluffyunicorn59803
Onsider the following reaction at equilibrium:
C(s)+H2O(g)⇌CO(g)+H2(g)
Predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances.
A. C is added to the reaction mixture.
B. H2Ois condensed and removed from the reaction mixture.
C. CO is added to the reaction mixture.
D. H2 is removed from the reaction mixture.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Onsider the following reaction at equilibrium:
C(s)+H2O(g)⇌CO(g)+H2(g)
Pre...
C(s)+H2O(g)⇌CO(g)+H2(g)
Pre...
Questions

Physics, 04.09.2020 07:01

Biology, 04.09.2020 07:01

Biology, 04.09.2020 07:01

Mathematics, 04.09.2020 07:01

Mathematics, 04.09.2020 07:01



Health, 04.09.2020 07:01


Mathematics, 04.09.2020 07:01

Social Studies, 04.09.2020 07:01


Biology, 04.09.2020 07:01

Mathematics, 04.09.2020 07:01


Mathematics, 04.09.2020 07:01

Social Studies, 04.09.2020 07:01

Mathematics, 04.09.2020 07:01


Mathematics, 04.09.2020 07:01

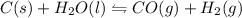
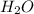 is condensed and removed from the reaction mixture.
is condensed and removed from the reaction mixture.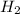 is removed from the reaction mixture.
is removed from the reaction mixture.

