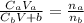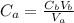Chemistry, 10.03.2020 04:00 dsaefong00
A 23.74-mL volume of 0.0981 M NaOH was used to titrate 25.0 mL of a weak monoprotic acid solution to the stoi- chiometric point. Determine the molar concentration of the weak acid solution. Express your answer to the correct number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

You know the right answer?
A 23.74-mL volume of 0.0981 M NaOH was used to titrate 25.0 mL of a weak monoprotic acid solution to...
Questions

Mathematics, 21.12.2019 23:31


Biology, 21.12.2019 23:31

History, 21.12.2019 23:31


Mathematics, 21.12.2019 23:31


Mathematics, 21.12.2019 23:31

English, 21.12.2019 23:31

Mathematics, 21.12.2019 23:31

History, 21.12.2019 23:31

Physics, 21.12.2019 23:31


History, 21.12.2019 23:31





Mathematics, 22.12.2019 00:31

Mathematics, 22.12.2019 00:31