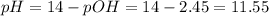Chemistry, 10.03.2020 03:30 morganmattal
Calculate the pH and concentrations of CH 3 NH 2 and CH 3 NH + 3 in a 0.0317 M methylamine ( CH 3 NH 2 ) solution. The K b of CH 3 NH 2 is 4.47 × 10 − 4 .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Calculate the pH and concentrations of CH 3 NH 2 and CH 3 NH + 3 in a 0.0317 M methylamine ( CH 3 NH...
Questions

Mathematics, 02.11.2020 07:20



Physics, 02.11.2020 07:20

Mathematics, 02.11.2020 07:20

Social Studies, 02.11.2020 07:20

Mathematics, 02.11.2020 07:20


SAT, 02.11.2020 07:20


Arts, 02.11.2020 07:20





Mathematics, 02.11.2020 07:20

English, 02.11.2020 07:20


Mathematics, 02.11.2020 07:20

![K_{b} = \frac{[CH_{3}NH_{3}^{+}][OH^{-}]}{[CH_{3}NH_{2}]}](/tpl/images/0539/8001/0cb42.png)
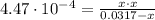
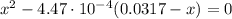 (2)
(2) ![pOH = -log [OH^{-}] = -log (0.00355) = 2.45](/tpl/images/0539/8001/ff2f0.png)