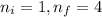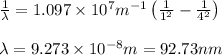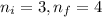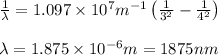Chemistry, 10.03.2020 02:57 urstruulyemily
In the emission spectrum of hydrogen the transitions observed in this experiment are in the visible region corresponding to the Balmer series in other series emmission lines are present in different regions of the eletromagnetic spectrum
Calculate the wavelenth of the n=4 to n=1 and the n=4 to n=3 transitions. Indicate in which regions of the electromagnetic spectrum these transitions would occur.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
In the emission spectrum of hydrogen the transitions observed in this experiment are in the visible...
Questions

Health, 14.09.2019 23:10


English, 14.09.2019 23:10

Chemistry, 14.09.2019 23:10





Mathematics, 14.09.2019 23:10




Mathematics, 14.09.2019 23:10

Chemistry, 14.09.2019 23:10


Mathematics, 14.09.2019 23:10


English, 14.09.2019 23:10


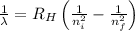
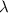 = Wavelength of radiation
= Wavelength of radiation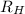 = Rydberg's Constant =
= Rydberg's Constant = 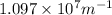
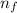 = Higher energy level
= Higher energy level 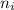 = Lower energy level
= Lower energy level