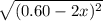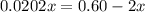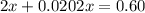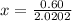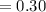Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

You know the right answer?
At 2000°C, the equilibrium constant for the reaction below is Kc = 4.10 ´ 10–4 . If 0.600 moles of N...
Questions

Mathematics, 11.10.2019 02:10

Mathematics, 11.10.2019 02:10


Biology, 11.10.2019 02:10









Computers and Technology, 11.10.2019 02:10







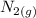 is going to be 0.30M
is going to be 0.30M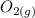 ⇄ 2
⇄ 2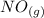
![K_{c}=\frac{[products]^{stoichiometric coefficient} }{[reactants]^{stoichiometric coefficient} }](/tpl/images/0539/5556/af056.png)
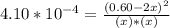
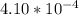 =
= 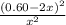
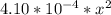 =
= 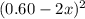
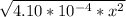 =
=